Evaluating Environmental Exposures
Overall Process
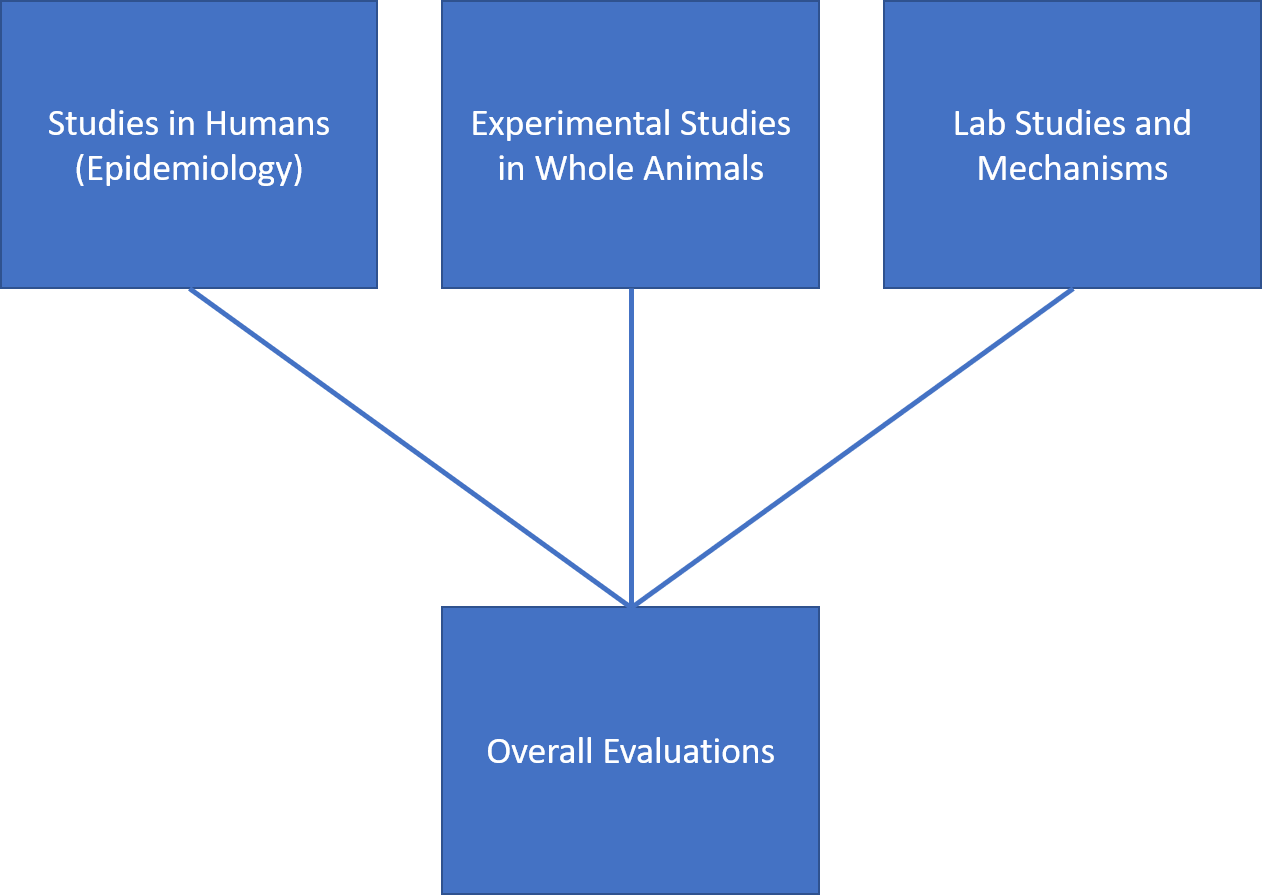
Like hundreds of other environmental agents, EMF has undergone extensive expert review with respect to potential health risks associated with exposure. These evaluations use a ‘weight-of-evidence’ methodology in which a panel of multi-disciplinary scientific experts considers the full body of research according to the general process flow shown in Figure 1 . By its very name this process must await the accumulation over years of a critical volume of research that permits a balanced and objective evaluation according to established criteria.
Epidemiology
Epidemiology, represented in the upper left box in Figure 1 , is the study of patterns and determinants of disease within human populations. Its most important advantage is that data are obtained about real people under actual exposure conditions. A disadvantage is that sampling and studying people is not a neat and clean process like separating cells into exposed and unexposed culture dishes in a laboratory.
The most commonly used study design in EMF epidemiology involves the selection of individuals from a defined geographic region, within a given age bracket, diagnosed with the disease or outcome of interest within a defined interval of calendar time; we can call this group the ‘cases’. A second group, referred to as ‘controls’, consists of subjects representing the same demographic, but who are disease-free. Each individual from both groups is assigned an exposure score by any of various methodologies (which will not be described here).
The analytical objective is to compare the EMF exposure profiles of the two groups, that is, how EMF exposure is distributed across both groups. If statistical analyses indicate that the two profiles are about equal, then one concludes that the disease was not associated with EMF. On the other hand, if the exposure profile for the cases is clearly greater than for the controls, then the analysis could suggest that the disease and exposure are ‘positively associated’ with one another. Epidemiology results are most often reported as ‘relative risks’ (often abbreviated as RR), which is a value that reflects the occurrence of disease in an exposed population compared to that disease’s occurrence in a population with comparatively low exposures (often referred to for simplicity as an ‘unexposed’ population). The bookmark icon below provides more information on relative risk.
A positive association means that the exposure is correlated or somehow related to the disease, not necessarily its direct cause. For example, a positive association could also represent an artifact owing to how the study population was sampled. Sampling human populations and soliciting their participation in a study such that the two groups of subjects are demographically equivalent is burdened with challenges. Thus, unequal sampling could skew the data to produce an impression of an association when one does not actually exist. Alternatively, the exposure under study may be masking the effect of another, yet unidentified, environmental factor with which it is highly correlated. This is why drawing broad conclusions about an exposure’s risk or lack of risk cannot be based on a single or small handful of studies, but requires judgments based on a sufficiently large body of evidence.
As an example, a few early EMF epidemiology studies suggested a possible link of residential magnetic fields with brain cancer in children. With time additional studies of brain cancer were completed that were not supportive of the early findings. Finally, in 2010, an analysis was conducted pooling the childhood brain cancer data from all 10 available studies. The investigators concluded, “Taken as a whole, our results provide little evidence for an association between ELF-MF [extremely-low-frequency magnetic fields] exposure and childhood brain tumors.” We cannot say for sure what the entire basis was for this series of observations; possibly, the quality of studies improved over time that minimized artifacts present in the earlier studies. In either case, the data accumulated to a point that a positive association between magnetic fields and childhood brain cancer, suggested by the earlier studies, was no longer apparent.
Studies in Whole Animals
The second major stream of evidence comes from studies of whole animals (usually mice and/or rats). With respect to cancer outcomes, the experiments are long-term, with many lasting for most or all of the animals’ lifespan; such studies are often referred to as ‘bioassays’. The animals are split into exposure groups, with one group remaining unexposed to serve as a control group. In the magnetic field bioassays that were conducted, the exposures were many times the levels typically experienced by humans, extending up to 10,000- 50,000 mG (our typical exposures are about 1-10,000 times lower). Exposure parameters are selected on factors such as: scaling based on size, field coupling, maximum expected exposures, and animal biological characteristics.
One may question the applicability of experiments in rodents to humans, but two factors should be borne in mind. Despite their external appearance, rats and mice are genetically very similar to humans. Secondly, rodent bioassays have an excellent track record in identifying exposures carcinogenic to humans. The International Agency for Research on Cancer (IARC, discussed later) has evaluated nearly 1,000 exposures for their carcinogenic potential and published its results over the past three decades in a series of detailed reports, called monographs. In the latest version of its preamble to its monographs (2006), IARC states that: “All known human carcinogens that have been studied adequately for carcinogenicity in experimental animals have produced positive results in one or more animal species.” Many bioassays of animals exposed to magnetic fields have by now been conducted with a uniform lack of effects on cancer development (including leukemia), which strongly suggests a lack of carcinogenicity in humans.
Laboratory Studies and Mechanisms
The third element of a risk evaluation includes (1) in vitro studies, meaning laboratory studies of cells and tissue placed in a laboratory culture dish and exposed to the agent of interest in a culture dish and (2) theoretical assessments of possible mechanisms of action, that is exploring how an agent such as a magnetic field may trigger a biological effect. These approaches are most useful when specific and validated effects have already been observed either in whole animals or in epidemiology studies. In a practical sense, without consistent or corroborating evidence in human and animal studies, it is not possible to get clues of effects that may occur in people or animals based only on observations in isolated cells or from theoretical analyses. For EMF, this third line of evidence has been unable to contribute research information or insights that would alter the conclusions based on epidemiologic and whole animal studies.
Thus, a risk evaluation relies on streams of evidence from different research disciplines and methodologies considered together and judged against established criteria that determine whether exposure to an environmental agent has the necessary and sufficient qualities to be considered a health risk.